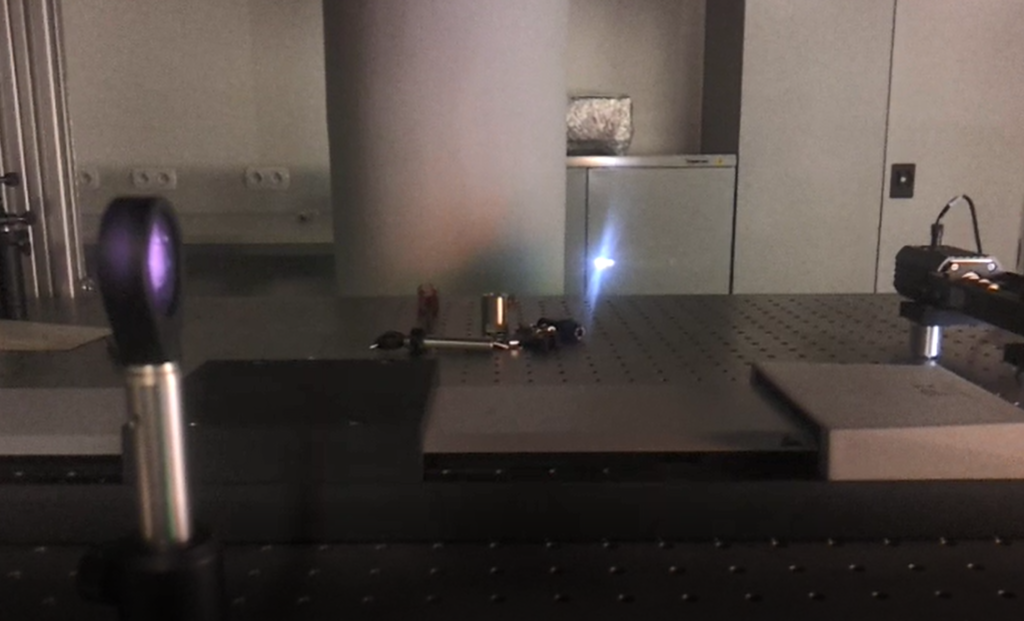
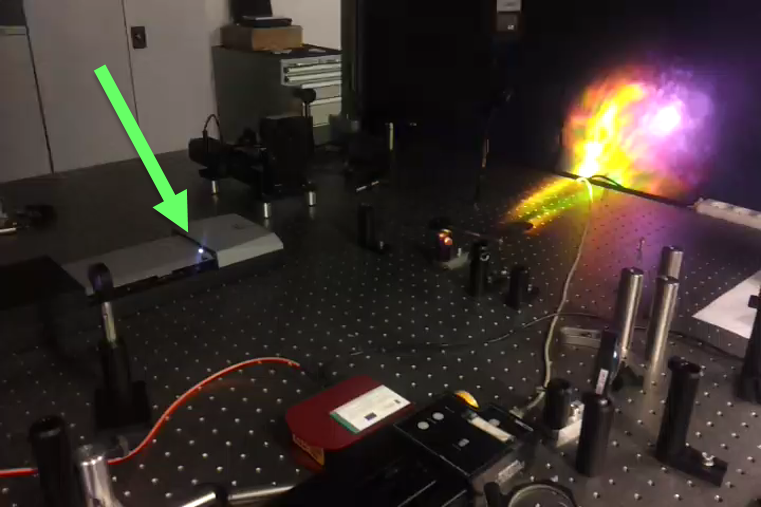
Laser-induced plasma
What’s plasma? Plasma is by far the most common form of matter!
Plasma in the stars and in the tenuous space between them makes up over 99% of the visible universe and perhaps most of that which is not visible. Plasma is partially or fully ionized gas comprising molecules, atoms, ions, electrons and photons. The concept of this state of matter was introduced by I. Langmuir and L. Tonks in 1929.
At temperatures below ~10 eV one refers to plasma as low-temperature or cold. At T>10 eV full ionization of neutral particles occures and plasma is called high-temperature or hot.The main difference between plasma and gas is the presence of charged particles in plasma, i.e. electrons and ions what makes plasma electrically conductive. Energy needed to strip electrons from atoms to make plasma can be of various origins: thermal, electrical, or light (ultraviolet light or intense visible light from a laser).
With insufficient sustaining power, plasmas recombine into neutral gas.
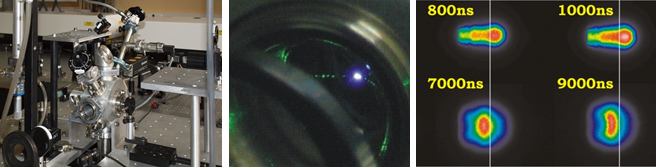
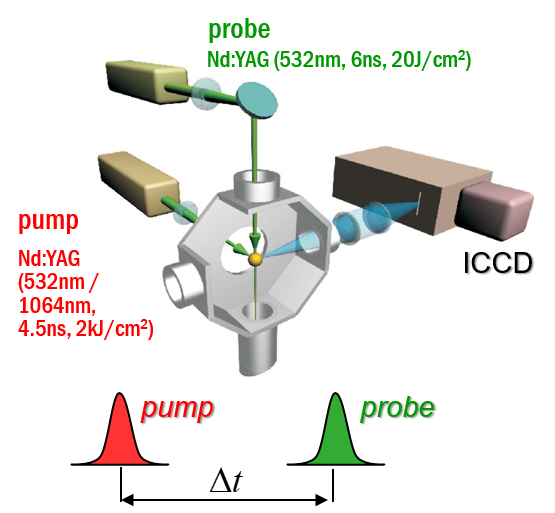
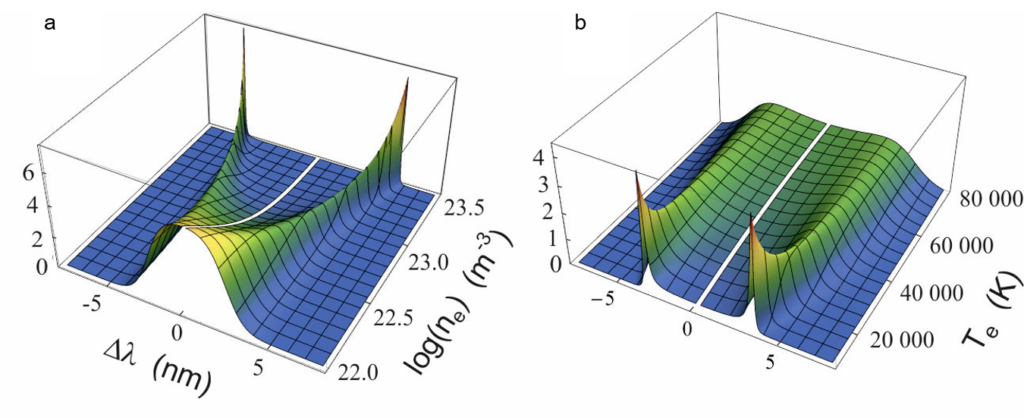
We perform measurements of laser-induced plasma in order to extract its key properties. High-resolution spectra are recorded with pump-probe laser configuration and a sensitive spectrometer equipped with precisely gated image-intensified CCD camera. Spectral analysis unravels electron density and local temperature. These parameters are usually determined using emission spectroscopy, which is the easiest and non-invasive method to perform. It is, however, characterized by certain limitations, such as spatial summation of the emitted radiation, or the need to make certain assumptions about thermodynamic equilibria when interpreting the spectra. To break free from these limitations, the main method we use is Thomson scattering, which is light scattering on free electrons in the plasma. Compared to emission spectroscopy, Thomson scattering is characterized by good spatial resolution and easy interpretation of the obtained spectra. By studying the light from the second pulse, scattered on the plasma, the electron temperature and density can be determined accurately. The parameter values obtained by this method can be easily compared with their values obtained from other methods.
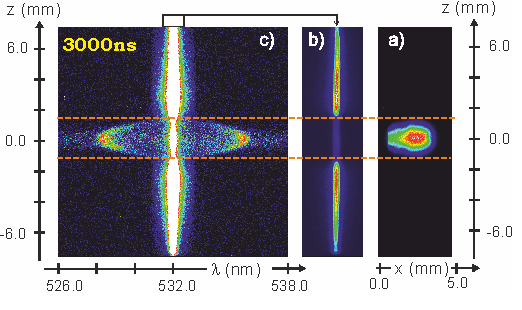
(b) scattered radiation recroded with the ICCD, (c) zoomed-in spectrum.
Laser-Induced Breakdown Spectroscopy (LIBS)
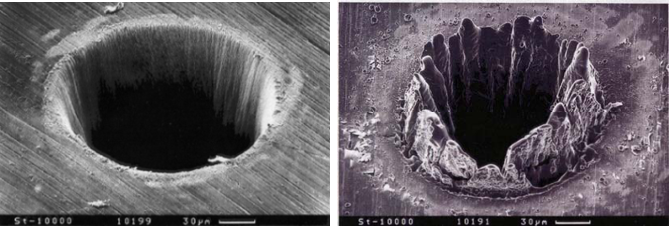
Modern lasers, thanks to the ability to generate ultra-short pulses, allow you to easily create plasma, either in gases or at the surfaces of material objects. This allows for the observation of characteristic optical spectra that reveal the chemical composition of the tested material. This method is called LIBS and is used many research laboratories for testing chemical composition and precision removal of the targeted material. It’s even used on Mars!